| Mathieu SauthierCatalyse et Chimie Moléculaire |
| Teléfono: | (+33) 03 74 95 13 84 |
| Correo: | Esta dirección de correo electrónico está siendo protegida contra los robots de spam. Necesita tener JavaScript habilitado para poder verlo. |
| Dirección: | Ecole Nationale Supérieure de Chimie de Lille Cité Scientifique, Bâtiment C7 - BP 90108 59652 Villeneuve d'Ascq Cedex France |
Education
| 2011 | Professor at the University of Lille 1 (France) – IUT « A » Unité de Catalyse et de Chimie du Solide (UCCS) Homogeneous catalysis – Chemistry of CO and 1,3-butadiene – conversion of bioresources (polyols) |
| 2002-2011 | Assistant professor at the University of Lille 1 (France) – IUT « A » HDR (Habilitation – 22th of November 2010) Unité de Catalyse et de Chimie du Solide (UCCS) |
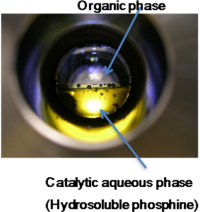
| 2001-2002 | Postdoc at the Universiteit van Amsterdam (UVA) in Pr Piet W.N.M. van Leeuwen’s group. Supervision by the Pr. Joost Reek. Synthesis of amphiphilic phosphorous based ligands – Hydroformylation under biphasic aqueous phase |
| 1998-2001 | Ph.D. at the University of Rennes 1 (France) Director : Pr. R. Réau. « New 1,2-diiminophosphoranes and (2-pyridyl)phospholes: New mixed N,N et P,N ligands for homogeneous catalysis ». |
| 1997-1998 | Master from the University of Burgundy (Dijon – France). Director : Pr. R. Guilard. « Synthesis of porphyrins as biomimetic oxygen carriers » |
Current research interest :
Homogeneous catalysis toward organic synthesis
Atom efficiency – greener chemistry – polyols valorization
|
Chemistry of carbon monoxide Synthesis of compounds with a carbonyle function |
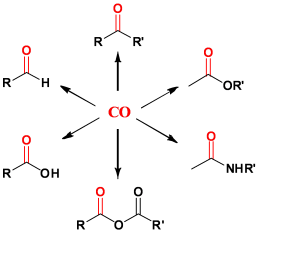 |
| (ketoesters, amides, esters, lactones, ketones, diketones ... ) Carbonylative suzuki coupling reactions Hydroformylation Alkoxycarbonylation Hydroesterification ... Catalyst improvements / screening: Catalytic activity (TOF), selectivity and life time (TON). |
|
 |
Saving synthetic and purification steps Domino carbonylative reactions: multiple bonds construction under “One pot” conditions. The construction of elaborated molecules is one step is targeted. Choice of catalysts / reactions conditions and substrates are carefully chosen in order to limit the importance of side reactions or catalyst poisoning. |
| Example of studied domino reaction: | |
 4 new bonds in a “one pot” procedure ! 4 new bonds in a “one pot” procedure ! |
|
|
Chemistry of 1,3-butadiene
|
|
Book chapters:
BOOK CHAPTER : Carbohydrate Chemistry : Vol. 40 ; 2014 – Royal Society of Chemistry - Ed. : A. P. Rauter, T. Lindhorst, Y. Queneau
Chapter 5 : “From conventional to greener catalytic approaches for carbohydrates authentification”
M. Sauthier, A. Mortreux, I. Suisse
BOOK CHAPTER : Science of Synthesis: C-1 Building Blocks in Organic Synthesis; Vol. 1; 2014 - Thieme – Ed. P. W. N. M. van Leeuwen
Chapter 1.1.4 : “Nonconventional Reaction Media: Hydroformylation, Carbonylation, and Hydrocarbonylation of Alkenes”
A. Mortreux, M. Sauthier, E. Monflier, S. Tilloy
Bibliographie
Articles
Publicaciones en la base de datos: 50 (desde 2005)
2023
- Efficient and sustainable one‐pot synthesis of α‐carbonyl homoallylic alcohols from benzaldehyde and allylic alcohols using both NHC and nickel catalysts.
Homrani Yasmina, Mouhsine Bouchaib, Béthegnies Aurélien, El Amrani Mohamed Amin, Karim Abdallah, Suisse Isabelle, Sauthier Mathieu.
ChemCatChem, (2023) DOI
DOI
- Catalytic hydroesterification of lignin: a versatile and efficient entry into fully biobased tunable materials.
Buhaibeh Ruqaya, Richard Tiphaine, Gauvin Régis M., Sauthier Mathieu, Dumont Clément.
Green Chemistry, vol. 25, pg. 1842-1851 (2023) DOI
DOI
2022
- Substituted N-heterocyclic carbene PEPPSI-type palladium complexes with different N-coordinated ligands: Involvement in the direct C H bond activation of heteroarenes derivatives with aryl bromide and their antimicrobial, anti-inflammatory and antioxidant activities.
Slimani I., Boubakri L., Özdemir N., Mansour L., Özdemir I., Gürbüz N., YAŞAR S., Sauthier M., Hamdi N.
Inorganica Chimica Acta, vol. 532, pg. 120747 (2022) DOI
DOI
- Synthesis, characterization and in vitro bioactivity studies of isoindolin‐1‐3‐phosophonate compounds.
Jelali Hamida, Al Nasr Ibrahim S., Koko Waleed S., Khan Tariq A., Deniau Eric, Sauthier Mathieu, Alresheedi Faisal, Hamdi Naceur.
Journal of Heterocyclic Chemistry, vol. 59, pg. 493-506 (2022) DOI
DOI
- The Selective Nickel-Catalyzed N-Allylation of C3-Unprotected Indoles under Mild and Clean Conditions
.
Mouhsine Bouchaib, Karim Abdallah, Dumont Clément, Saint Pol Anthony, Suisse Isabelle, Sauthier Mathieu.
European Journal of Organic Chemistry, vol. 2022 (2022) DOI
DOI
- Ruthenium(II) complexes bearing benzimidazole-based N-heterocyclic carbene (NHC) ligands as potential antimicrobial, antioxidant, enzyme inhibition, and antiproliferative agents.
Boubakri Lamia, Chakchouk-Mtiba Ahlem, Naouali Olfa, Mellouli Lotfi, Mansour Lamjed, Özdemir Ismail, Yaser Sedat, Sauthier Mathieu, Hamdi Naceur.
Journal of Coordination Chemistry, vol. 75, pg. 645-667 (2022) DOI
DOI
- Synthesis and crystal structures of palladium complexes based on α-amino-oximes derived from (R)-limonene and their application in allylic alkylation of 1,3-dioxo compounds.
Homrani Yasmina, El Amrani Mohamed Amin, Loxq Pauline, Capet Frédéric, Suisse Isabelle, Sauthier Mathieu.
Comptes Rendus. Chimie, vol. 25, pg. 125-135 (2022) DOI
DOI
2021
- Reductive Hydroformylation of Isosorbide Diallyl Ether.
Ternel Jérémy, Lopes Adrien, Sauthier Mathieu, Buffe Clothilde, Wiatz Vincent, Bricout Hervé, Tilloy Sébastien, Monflier Eric.
Molecules, vol. 26, pg. 7322 (2021) DOI
DOI
- Synthesis and crystal structure of a new chiral α-aminooxime nickel(II) complex.
Homrani Yasmina, Dahdouh Abdelaziz, El Amrani Mohamed Amin, Loxq Pauline, Capet Frédéric, Suisse Isabelle, Sauthier Mathieu.
Acta Crystallographica Section E Crystallographic Communications, vol. 77, pg. 1116-1119 (2021) DOI
DOI
- Palladium catalyzed telomerization of bio-based polyols with atmospheric pressure bubbling butadiene.
Peruzzo Deborah, Drelon Mathieu, Dumont Clément, Mortreux André, Suisse Isabelle, Sauthier Mathieu.
Molecular Catalysis, vol. 502, pg. 111369 (2021) DOI
DOI
- Efficient and Clean Nickel Catalyzed α‐Allylation Reaction of Nitriles.
Mouhsine Bouchaib, Karim Abdallah, Dumont Clément, Suisse Isabelle, Sauthier Mathieu.
Advanced Synthesis < Catalysis, vol. 363, pg. 1457-1462 (2021) DOI
DOI
2020
- Enantioselective Nickel Catalyzed Butadiene Hydroalkoxylation with Ethanol: from Experimental Results to Kinetics Parameters.
Mifleur Alexis, Suisse Isabelle, Mortreux André, Sauthier Mathieu.
Catalysis Letters, vol. 151, pg. 27-35 (2020) DOI
DOI
- An Efficient Synthesis of Phthalimides and Their Biological Activities.
Jelali H., Mansour L., Deniau E., Sauthier M., Hamdi N.
Polycyclic Aromatic Compounds, vol. 42 (4), pg. 1806*18013 (2020) DOI
DOI
- The salt-free nickel-catalysed α-allylation reaction of ketones with allyl alcohol and diallylether.
Mouhsine Bouchaib, Karim Abdallah, Dumont Clément, Sauthier Mathieu.
Green Chemistry, vol. 22, pg. 950-955 (2020) DOI
DOI
- Visible-Light-Driven CarboxyLic Amine Protocol (CLAP) for the Synthesis of 2-Substituted Piperazines under Batch and Flow Conditions.
Gueret Robin, Pelinski Lydie, Bousquet Till, Sauthier Mathieu, Ferey Vincent, Bigot Antony.
Organic Letters, vol. 22, pg. 5157-5162 (2020) DOI
DOI
- Photocatalyzed Amidoarylation of 1,3‐Butadiene.
Gosset Cyrille, Moncomble Aurélien, Dumont Clément, Pellegrini Sylvain, Bousquet Till, Sauthier Mathieu, Pélinski Lydie.
Advanced Synthesis < Catalysis, (2020) DOI
DOI
2019
- Towards Selective Syntheses of Octadienylethers from the Butadiene Palladium‐catalyzed Telomerization with Polyols in Aqueous Biphasic Medium.
Drelon Mathieu, Mérel Delphine S., Mortreux André, Suisse Isabelle, Sauthier Mathieu.
ChemCatChem, vol. 11, pg. 1742-1746 (2019) DOI
DOI
- The Palladium‐Catalyzed Carboxytelomerization of Butadiene with Agrobased Alcohols and Polyols.
Wilson Emma, Dumont Clément, Drelon Mathieu, Suisse Isabelle, Penverne Christophe, Sauthier Mathieu.
ChemSusChem, vol. 12 (11), pg. 2457-2461 (2019) DOI
DOI
- Catalytic dehydration of sorbitol to isosorbide in the presence of metal tosylate salts and metallized sulfonic resins.
Dussenne Corentin, Wyart Hervé, Wiatz Vincent, Suisse Isabelle, Sauthier Mathieu.
Molecular Catalysis, vol. 463, pg. 61-66 (2019) DOI
DOI
2018
- The Palladium-Catalyzed Carbonylative Telomerization Reaction with Phenols, Polyphenols and Kraft Lignin.
Dumont Clément, Belva Frederic, Gauvin Regis M., Sauthier Mathieu.
ChemSusChem, vol. 11, pg. 3917-3922 (2018) DOI
DOI
- Efficient in situ N-heterocyclic carbene palladium(ii) generated from Pd(OAc)2 catalysts for carbonylative Suzuki coupling reactions of arylboronic acids with 2-bromopyridine under inert conditions leading to unsymmetrical arylpyridine ketones: synthesis, characterization and cytotoxic activities.
Touj Nedra, Al-Ayed Abdullah S., Sauthier Mathieu, Mansour Lamjed, Harrath Abdel Halim, Al-Tamimi Jamil, Özdemir Ismail, Yaşar Sedat, Hamdi Naceur.
RSC Advances, vol. 8, pg. 40000-40015 (2018) DOI
DOI
- Palladium-Catalyzed Functionalization of Kraft Lignin: Ether Linkages through the Telomerization Reaction.
Dumont Clément, Gauvin Régis M., Belva Frédéric, Sauthier Mathieu.
ChemSusChem, vol. 11, pg. 1649-1655 (2018) DOI
DOI
2017
- Synthesis of isosorbide: an overview of challenging reactions.
Dussenne C., Delaunay T., Wiatz V., Wyart H., Suisse I., Sauthier M.
Green Chemistry, vol. 19, pg. 5332-5344 (2017) DOI
DOI
- Deciphering the Mechanism of the Nickel-Catalyzed Hydroalkoxylation Reaction: A Combined Experimental and Computational Study.
Mifleur Alexis, Mérel Delphine S., Mortreux André, Suisse Isabelle, Capet Frédéric, Trivelli Xavier, Sauthier Mathieu, Macgregor Stuart A.
ACS Catalysis, vol. 7, pg. 6915-6923 (2017) DOI
DOI
- Synthesis of C4 chain glyceryl ethers via nickel-catalyzed butadiene hydroalkoxylation reaction.
Mifleur Alexis, Mortreux André, Suisse Isabelle, Sauthier Mathieu.
Molecular Catalysis, vol. 427, pg. 25-30 (2017) DOI
DOI
- Nickel-Catalyzed α-Allylation of Aldehydes and Tandem Aldol Condensation/Allylation Reaction with Allylic Alcohols.
Bernhard Yann, Thomson Brodie, Ferey Vincent, Sauthier Mathieu.
Angewandte Chemie International Edition, vol. 56, pg. 7460-7464 (2017) DOI
DOI
2016
- Synthesis of Short-Chain Alkenyl Ethers from Primary and Bio-sourced Alcohols via the Nickel-Catalyzed Hydroalkoxylation Reaction of Butadiene and Derivatives.
Mifleur Alexis, Ledru Hélène, Lopes Adrien, Suisse Isabelle, Mortreux André, Sauthier Mathieu.
Advanced Synthesis < Catalysis, vol. 358, pg. 110-121 (2016) DOI
DOI
- Telomerization of 1,3-butadiene with glycerol carbonate and subsequent ring-opening lactone co-polymerization.
Ibn El Alami Mohammed Samir, Suisse Isabelle, Fadlallah Sami, Sauthier Mathieu, Visseaux Marc.
Comptes Rendus Chimie, vol. 19, pg. 299-305 (2016) DOI
DOI
- Nickel-Catalysed Bis-Allylation of Activated Nucleophiles with Allyl Alcohol.
Blieck Rémi, Azizi Mohamed Salah, Mifleur Alexis, Roger Maxime, Persyn Clément, Sauthier Mathieu, Bonin Hélène.
European Journal of Organic Chemistry, vol. 2016, pg. 1194-1198 (2016) DOI
DOI
- Nickel(0)-CatalyzedN-Allylation of Amides andp-Toluenesulfonamide with Allylic Alcohols under Neat and Neutral Conditions.
Azizi Mohamed Salah, Edder Youssef, Karim Abdallah, Sauthier Mathieu.
European Journal of Organic Chemistry, vol. 2016, pg. 3796-3803 (2016) DOI
DOI
2015
- Palladium-catalyzed hydroesterification of olefins with isosorbide in standard and Brønsted acidic ionic liquids.
Boulanger Jérôme, Seingeot Adeline, Léger Bastien, Pruvost Romain, Ibert Mathias, Mortreux André, Chenal Thomas, Sauthier Mathieu, Ponchel Anne, Monflier Eric.
Catalysis Communications, vol. 69, pg. 143-146 (2015) DOI
DOI
- Biphasic Palladium-Catalyzed Hydroesterification in a Polyol Phase: Selective Synthesis of Derived Monoesters.
Pruvost Romain, Boulanger Jérôme, Léger Bastien, Ponchel Anne, Monflier Eric, Ibert Mathias, Mortreux André, Sauthier Mathieu.
ChemSusChem, vol. 8, pg. 2133-2137 (2015) DOI
DOI
2014
- Synthesis of 1,4:3,6-Dianhydrohexitols Diesters from the Palladium-Catalyzed Hydroesterification Reaction.
Pruvost Romain, Boulanger Jérôme, Léger Bastien, Ponchel Anne, Monflier Eric, Ibert Mathias, Mortreux André, Chenal Thomas, Sauthier Mathieu.
ChemSusChem, vol. 7 (11), pg. 3157-3163 (2014) DOI
DOI
- Transition Metal-Mediated Direct CH Arylation of Heteroarenes Involving Aryl Radicals.
Bonin Hélène, Sauthier Mathieu, Felpin François-Xavier.
Advanced Synthesis < Catalysis, vol. 356, pg. 645-671 (2014) DOI
DOI
2013
- Nickel-Catalysed Hydroalkoxylation Reaction of 1,3-Butadiene: Ligand Controlled Selectivity for the Efficient and Atom-Economical Synthesis of Alkylbutenyl Ethers.
Bigot Sandra, El Alami Mohammed Samir Ibn, Mifleur Alexis, Castanet Yves, Suisse Isabelle, Mortreux André, Sauthier Mathieu.
Chemistry - A European Journal, vol. 19, pg. 9785-9788 (2013) DOI
DOI
- Synthesis of α-Alkylated β-Ketoesters by Alkoxycarbonylation/Michael Addition Domino Reaction.
Wahl Benoit, Philipson Yann, Bonin Hélène, Mortreux André, Sauthier Mathieu.
The Journal of Organic Chemistry, vol. 78, pg. 1547-1552 (2013) DOI
DOI
2012
- Straightforward Synthesis of Allylated Keto Esters: The Palladium-Catalysed Haloketone Alkoxycarbonylation/ Allylation Domino Reaction.
Wahl Benoit, Giboulot Steven, Mortreux André, Castanet Yves, Sauthier Mathieu, Liron Frédéric, Poli Giovanni.
Advanced Synthesis < Catalysis, vol. 354, pg. 1077-1083 (2012) DOI
DOI
- Pd-catalyzed domino carbonylative–decarboxylative allylation: an easy and selective monoallylation of ketones.
Giboulot Steven, Liron Frédéric, Prestat Guillaume, Wahl Benoit, Sauthier Mathieu, Castanet Yves, Mortreux André, Poli Giovanni.
Chemical Communications, vol. 48, pg. 5889 (2012) DOI
DOI
- Effect of Chain Unsaturation on the Self-Association of Tri- and Tetraethylene Glycol Octyl Ethers Obtained by Butadiene Telomerization.
Lai Jonathan, Molinier Valérie, Sauthier Mathieu, Moity Laurianne, Castanet Yves, Mortreux André, Aubry Jean-Marie.
Langmuir, vol. 28, pg. 242-250 (2012) DOI
DOI
- A General and Efficient Method for the Alkoxycarbonylation of α-Chloro Ketones.
Wahl Benoit, Bonin Hélène, Mortreux André, Giboulot Steven, Liron Frédéric, Poli Giovanni, Sauthier Mathieu.
Advanced Synthesis < Catalysis, vol. 354, pg. 3105-3114 (2012) DOI
DOI
2011
- Telomerisation of 1,3-Butadiene with 1,4:3,6-Dianhydrohexitols: An Atom-Economic and Selective Synthesis of Amphiphilic Monoethers from Agro-Based Diols.
Lai Jonathan, Bigot Sandra, Sauthier Mathieu, Molinier Valérie, Suisse Isabelle, Castanet Yves, Aubry Jean-Marie, Mortreux André.
ChemSusChem, vol. 4, pg. 1104-1111 (2011) DOI
DOI
2010
- Organometallic catalysis: From concepts to selected applications.
Sauthier Mathieu, Zinck Philippe, Mortreux André.
Comptes Rendus Chimie, vol. 13, pg. 304-314 (2010) DOI
DOI
- Telomerisation of 1,3-butadiene with glycerol under aqueous biphasic conditions: Influence of the reaction conditions on the products distribution.
Bigot Sandra, Lai Jonathan, Suisse Isabelle, Sauthier Mathieu, Mortreux André, Castanet Yves.
Applied Catalysis A: General, vol. 382, pg. 181-189 (2010) DOI
DOI
- New Synthesis of Furans: the Rhodium-Catalysed Carbonylative Addition of Arylboronic Acids to Propargylic Alcohols/ Cyclisation Sequence.
Dheur Julien, Sauthier Mathieu, Castanet Yves, Mortreux André.
Advanced Synthesis < Catalysis, vol. 352, pg. 557-561 (2010) DOI
DOI
2009
- Palladium-Catalysed Carbonylative Cross-Coupling Reactions of Aryl Iodides and Vinyl Boron Derivatives as a Straightforward Route to Aryl Vinyl Ketones.
Castanet Yves, Sauthier Mathieu, Pirez Cyril, Dheur Julien, Mortreux André.
Synlett, vol. 2009, pg. 1745-1748 (2009) DOI
DOI
- Carbonylative 1,4-addition of potassium aryltrifluoroborates to vinyl ketones.
Sauthier Mathieu, Lamotte Nicolas, Dheur Julien, Castanet Yves, Mortreux André.
New Journal of Chemistry, vol. 33, pg. 969 (2009) DOI
DOI
- Aqueous hydroformylation reaction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity.
Legrand François-Xavier, Sauthier Mathieu, Flahaut Christophe, Hachani Johan, Elfakir Claire, Fourmentin Sophie, Tilloy Sébastien, Monflier Eric.
Journal of Molecular Catalysis A: Chemical, vol. 303, pg. 72-77 (2009) DOI
DOI
2008
- 1,4-Conjugate addition reaction catalyzed by a homogeneous rhodium catalyst entrapped in hydrophobized ordered mesoporous silica.
Handa Paul, Holmberg Krister, Sauthier Mathieu, Castanet Yves, Mortreux André.
Microporous and Mesoporous Materials, vol. 116, pg. 424-431 (2008) DOI
DOI
2005
- Rhodium Complexes Non-Covalently Bound to Cyclodextrins: Novel Water-Soluble Supramolecular Catalysts for the Biphasic Hydroformylation of Higher Olefins.
Sueur Benoît, Leclercq Loïc, Sauthier Mathieu, Castanet Yves, Mortreux André, Bricout Hervé, Tilloy Sébastien, Monflier Eric.
Chemistry - A European Journal, vol. 11, pg. 6228-6236 (2005) DOI
DOI
- Two-Phase Hydroformylation of Higher Olefins Using Randomly Methylated ?-Cyclodextrin as Mass Transfer Promoter: A Smart Solution for Preserving the Intrinsic Properties of the Rhodium/Trisulfonated Triphenylphosphine Catalytic System.
Leclercq Loïc, Sauthier Mathieu, Castanet Yves, Mortreux André, Bricout Hervé, Monflier Eric.
Advanced Synthesis < Catalysis, vol. 347, pg. 55-59 (2005) DOI
DOI